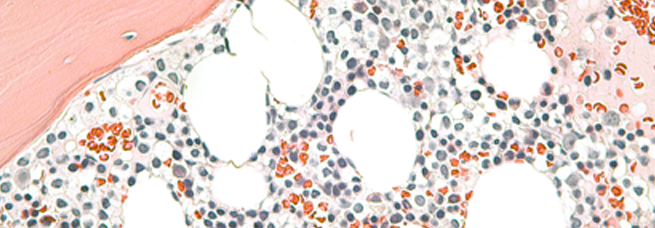
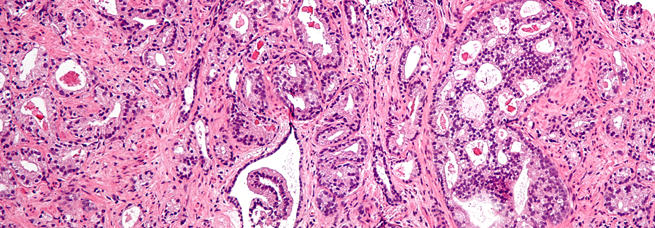
Pandolfi's laboratory has been seminal at elucidating the molecular mechanisms and the genetics underlying the pathogenesis of leukemias, lymphomas and solid tumors as well as in modeling these cancers.
Dr. Pandolfi and colleagues have characterized the function of the fusion oncoproteins and the genes involved in the chromosomal translocations of acute promyelocytic leukemia (APL), as well as of major tumor suppressors such PTEN and p53 and novel proto-oncogenes such POKEMON.
The elucidation of the molecular basis underlying APL pathogenesis has led to the development of novel and effective therapeutic strategies. As a result of these efforts, APL is now considered a curable disease. Novel therapeutic concepts have emerged from this work that are currently been tested in clinical trials.